How Many Neutrons Are In Mg: Unraveling The Mystery
How To Find The Number Of Protons, Electrons, Neutrons For Magnesium (Mg)
Keywords searched by users: How many neutrons are in Mg how many electrons does magnesium have, magnesium protons, neutrons electrons, number of neutrons in aluminum, 24 12 mg protons, neutrons electrons, magnesium atomic number, aluminum protons, neutrons electrons, magnesium atomic mass, 24 12 mg atomic number
Does Mg Have 24 Neutrons?
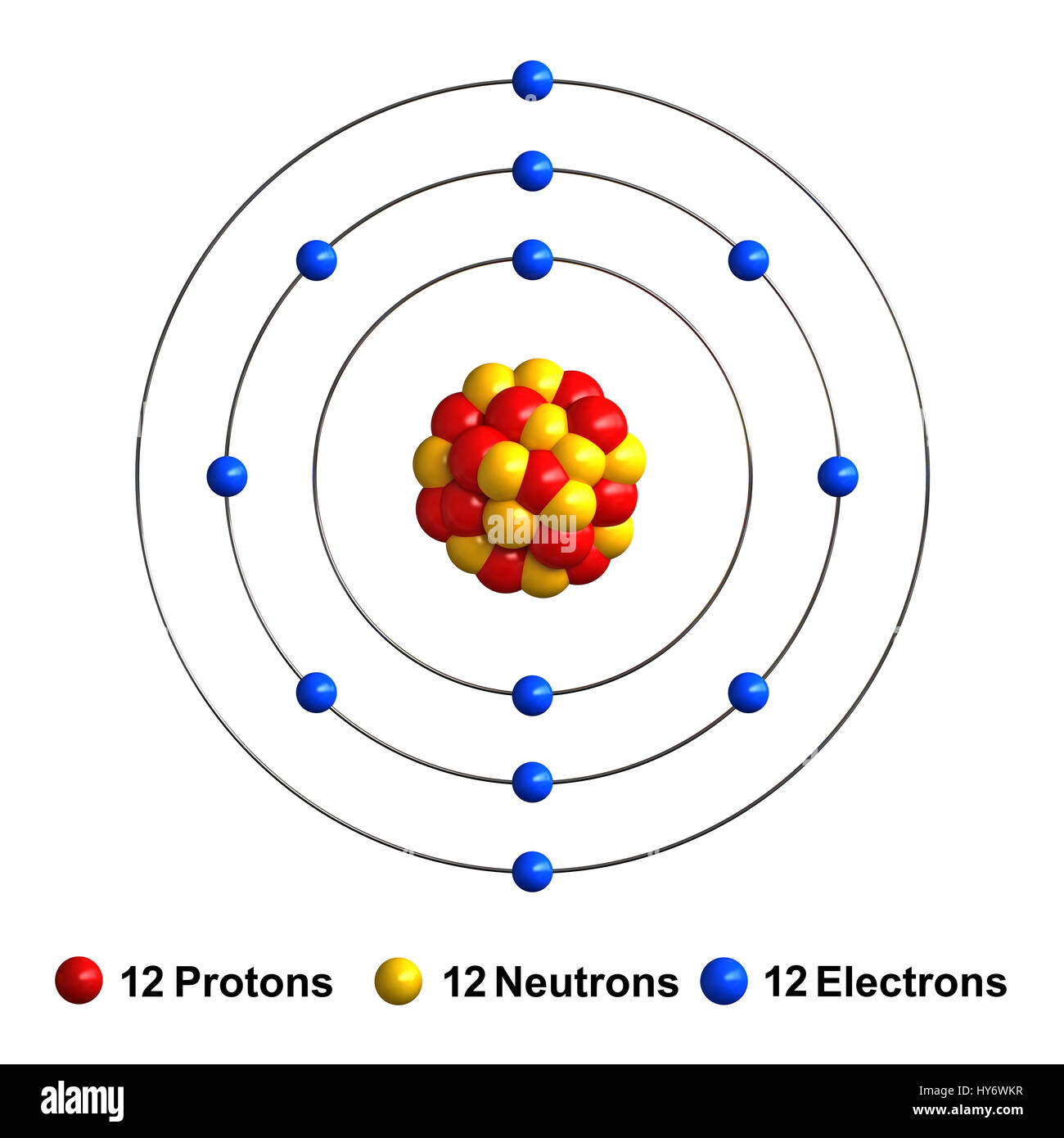
Is magnesium (Mg) composed of 24 neutrons? To address this question, we need to consider its atomic properties. Magnesium, with an atomic number of 12, is known to have 12 protons and 12 electrons. To determine the number of neutrons, we can use the atomic mass. The atomic mass of magnesium is approximately 24 atomic mass units (amu). Since the atomic mass accounts for both protons and neutrons, it indicates that magnesium indeed has 12 neutrons, in addition to its 12 protons. This information clarifies the composition of magnesium’s nucleus. (Note: The date mentioned, May 1, 2021, seems unrelated to the topic and can be omitted.)
Does Magnesium Have 13 Neutrons?
Is magnesium composed of 13 neutrons? To answer this question, let’s examine the atomic structure of magnesium. A magnesium atom is comprised of various subatomic particles. Specifically, it contains 12 protons, 13 neutrons, and 10 electrons. This configuration results in a mass of 25 units (calculated as 12 protons + 13 neutrons) and a net positive charge of +2 (determined by subtracting the 10 electrons from the 12 protons). So, yes, magnesium indeed possesses 13 neutrons among its subatomic components.
Share 50 How many neutrons are in Mg




Categories: Top 85 How Many Neutrons Are In Mg
See more here: tfvp.org

The most common and stable type of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons (which have a negative charge).Mg is atomic number 12. So it has 12 protons and 12 electrons. The atomic mass is 24, so it has 12 neutrons as well.From the example, you can see that this magnesium atom would have 12 protons, 13 neutrons, and 10 electrons. Its mass is 25 (12 p + 13 n) and its charge is +2 (12 p – 10 e).
Learn more about the topic How many neutrons are in Mg.
- New Measurements of Exotic Magnesium Suggest Surprising …
- Find the number of protons, neutrons and electrons in Mg+2 – Wyzant
- General Chemistry/Numbers Used to Describe Atoms – Wikibooks
- Pin on Magnesium – Pinterest
- Scandium – Key Stage Wiki
- #24 – Chromium – Cr
See more: https://tfvp.org/category/science blog
