What Is Emf Of A Cell: Exploring Electromotive Force In Batteries
Emf Of The Cell
Keywords searched by users: What is EMF of a cell what is emf of a cell in physics, how to calculate emf of a cell in chemistry, standard emf of cell, nernst equation for emf of a cell, Standard emf, internal resistance of a cell, Galvanic cell, emf full form
What Is Emf In Simple Words?
What is Electromotive Force (EMF) in simple terms? Electromotive force, often abbreviated as EMF, refers to the electrical push or driving force generated either by an electrochemical cell or as a result of changes in a magnetic field. It’s essentially the power that makes electricity flow. Think of a generator or a battery as devices that help transform energy from one type into electrical energy, which can then be used to power various devices or systems. In essence, EMF is the “oomph” that gets electrons moving in an electric circuit, allowing us to harness electricity for our needs.
What Is The Emf Of A Battery Cell?
What is the EMF of a battery cell? Electromotive force (EMF) is a fundamental concept in the world of batteries and electrical circuits. It is defined as the energy per unit charge that a battery provides when no current is flowing through it. This concept is often confused with terminal potential difference (V), which is also measured in volts but represents a different aspect of a battery’s behavior. EMF, denoted as ϵ, quantifies the energy (E) that the battery supplies to each coulomb of charge (Q) as it passes through the circuit. In essence, EMF represents the maximum potential energy available from the battery, while terminal potential difference (V) accounts for energy losses within the circuit due to factors like internal resistance. Understanding the distinction between EMF and terminal potential difference is crucial for comprehending how batteries function in practical applications.
Found 28 What is EMF of a cell




Categories: Share 100 What Is Emf Of A Cell
See more here: tfvp.org

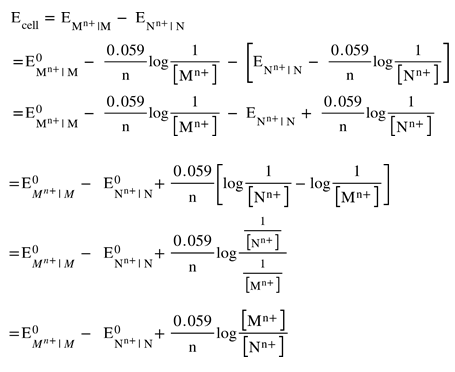
The electromotive force of a cell or EMF of a cell is the maximum potential difference between two electrodes of a cell. It can also be defined as the net voltage between the oxidation and reduction half-reactions. The EMF of a cell is mainly used to determine whether an electrochemical cell is galvanic or not.What Is Electromotive Force? Electromotive force is defined as the electric potential produced by either an electrochemical cell or by changing the magnetic field. EMF is the commonly used acronym for electromotive force. A generator or a battery is used for the conversion of energy from one form to another.Electromotive force (EMF) is equal to the terminal potential difference when no current flows. EMF and terminal potential difference (V) are both measured in volts, however they are not the same thing. EMF (ϵ) is the amount of energy (E) provided by the battery to each coulomb of charge (Q) passing through.
Learn more about the topic What is EMF of a cell.
- EMF of a Cell – Definition, Formulas, Calculation, Methods
- Electromotive Force – Definition, Formula, Unit, Difference – BYJU’S
- Physics A level revision resource – University of Birmingham
- Difference Between EMF and Voltage – Definitions, and Solved Examples
- EMF Formula: Equation, Explanation and Solved Examples
- Electromagnetic Fields and Cancer – NCI
See more: blog https://tfvp.org/category/science