Unlocking The Mystery: How Do Hydrogen Atom Orbitals Combine To Form Molecular Orbitals?
Chemistry Of Life Molecule (H2O): (Atoms Bond Of 2 Hydrogen \U0026 Oxygen For Air And Water)
Keywords searched by users: When the one is orbitals of two hydrogen atoms combine to form a hydrogen molecule how many molecular orbitals are formed when two hydrogen atoms combine with one oxygen atom what is the result, when two hydrogen atoms combine with one oxygen atom the result is a water molecule which is a, what is the hybridization of carbon in formaldehyde (h2co)?, how many p orbitals are left unhybridized on each carbon in acetylene (hcch)?
What Happens When Two Hydrogen Atoms Combine To Form A Molecule?
When two hydrogen atoms come together to create a molecule, they undergo a process known as hydrogen molecule formation. In this process, two individual hydrogen atoms, each having one electron located in a 1s orbital, combine. This union occurs through a covalent bond, where the two hydrogen atoms are drawn together by their mutual attraction to the same pair of electrons. This covalent bond can be symbolically represented either as a pair of “dots” or as a solid line between the two hydrogen atoms. As a result of this bonding, each hydrogen atom achieves a stable electron configuration similar to that of helium. This phenomenon, where hydrogen atoms combine to form molecules, is essential in understanding the behavior of matter and the formation of various chemical compounds in our universe.
How Many Molecular Orbitals Are Formed In H2?
To understand the formation of molecular orbitals in the H2 molecule, it’s crucial to consider its composition. H2 consists of two hydrogen (H) atoms, each of which possesses a 1s atomic orbital. When these two hydrogen atoms come together to form H2, the combination results in the creation of two distinct molecular orbitals. These molecular orbitals are known as bonding molecular orbitals and are characterized by lower energy levels compared to the original atomic orbitals from which they originated. As of January 29, 2023, this fundamental concept holds true, leading to the formation of two molecular orbitals in the H2 molecule.
When Two Atomic Orbitals Are Combined How Many Molecular Orbitals Are Formed?
When two atomic orbitals combine, they give rise to a total of two molecular orbitals. This phenomenon occurs because an equal number of atomic orbitals are involved in the process, resulting in an equivalent number of molecular orbitals being formed. This fundamental concept illustrates the direct relationship between the number of atomic orbitals combining and the number of resulting molecular orbitals, ensuring a balanced outcome in molecular orbital formation.
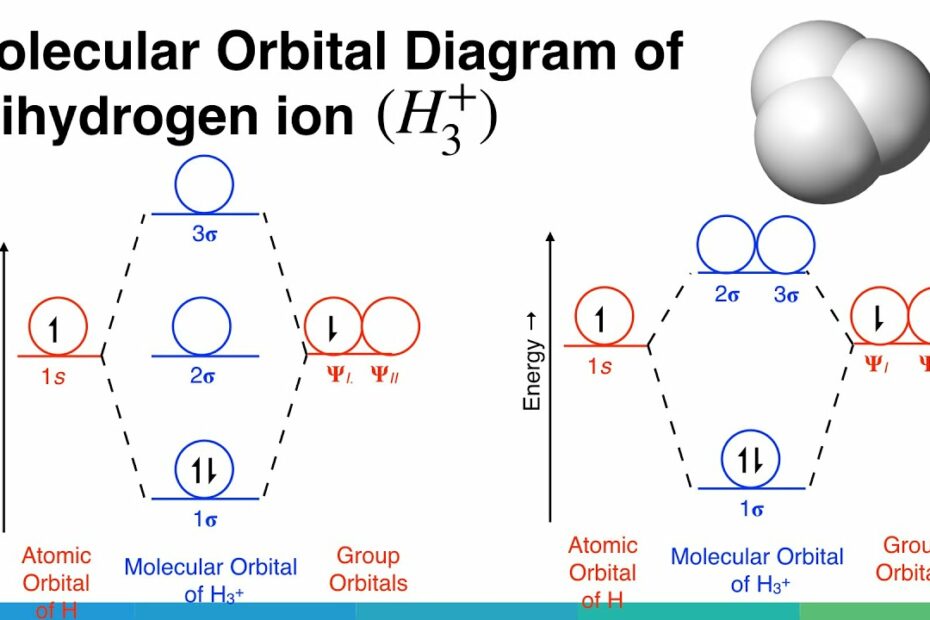
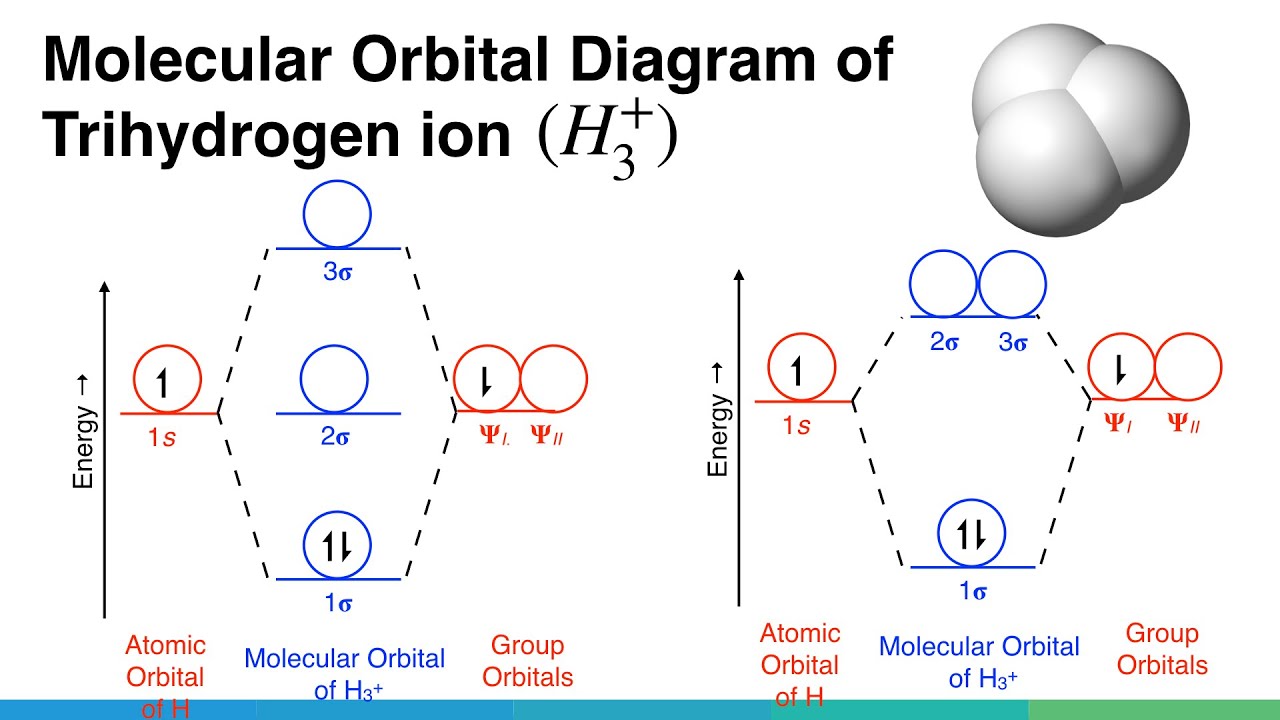
Collect 8 When the one is orbitals of two hydrogen atoms combine to form a hydrogen molecule how many molecular orbitals are formed
See more here: tfvp.org

So if you have two 1s atomic orbitals you can only make two molecular orbitals from them. This is the First Principle. According to MO Theory, the two molecular orbitals that form are called s (sigma = bonding) and s* (sigma star = antibonding).A hydrogen molecule forms from two hydrogen atoms, each with one electron in a 1 s orbital. The two hydrogen atoms are attracted to the same pair of electrons in the covalent bond. The bond is represented either as a pair of “dots” or as a solid line. Each hydrogen atom acquires a helium-like electron configuration.The molecule H2 is composed of two H atoms. Both H atoms have a 1s orbital, so when bonded together, there are therefore two molecular orbitals. Bonding molecular orbitals are lower energy than the atomic orbitals from which they were formed.
Learn more about the topic When the one is orbitals of two hydrogen atoms combine to form a hydrogen molecule how many molecular orbitals are formed.
- Mo Theory
- Covalent Bond – an overview | ScienceDirect Topics
- Pictorial Molecular Orbital Theory – Chemistry LibreTexts
- When two atomic orbitals combine, they form: – Toppr
- Molecular Orbital Theory
- Hydrogen Bonding – Chemistry LibreTexts
See more: https://tfvp.org/category/science blog