Where Is The Peptide Bond Located In This Dipeptide: Unveiling The Chemical Connection
Peptides And Peptide Bonds | Amino Acids, Dipeptides, Oligopeptides, Polypeptides | Biochemistry
Keywords searched by users: Where is the peptide bond located in this dipeptide
What Is The Peptide Bond In A Dipeptide?
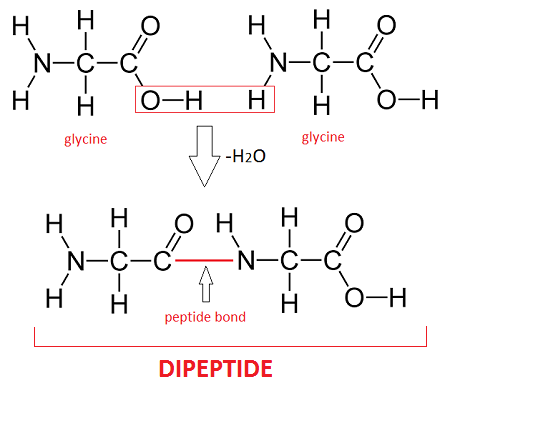
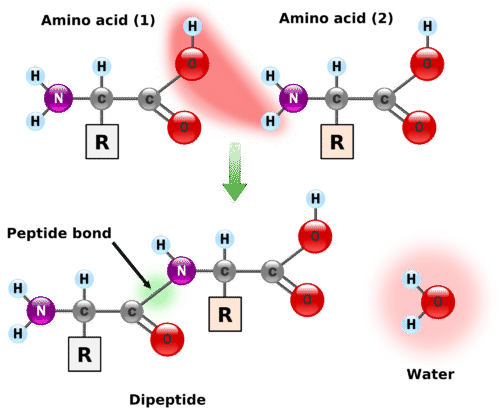
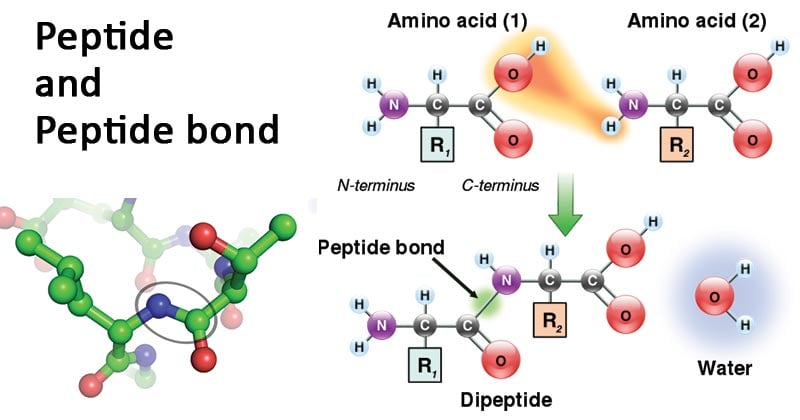
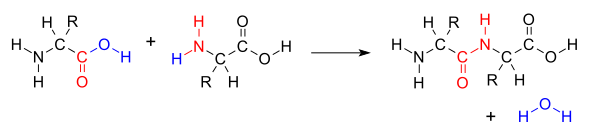
A dipeptide is a molecule formed when two amino acids undergo a condensation reaction, where a peptide bond (also known as an amide bond) is created. This peptide bond forms between the amine nitrogen of one amino acid and the carboxyl carbon of another amino acid. This chemical linkage is crucial for connecting individual amino acids to construct larger protein structures. Therefore, a dipeptide represents the outcome of this bonding process, where two amino acids are joined together through the formation of a peptide bond. This fundamental biochemical process is vital in understanding the structure and function of proteins. [Date: August 13, 2020]
Where Is Peptide Bond Found?
A peptide bond is a fundamental type of covalent chemical bond with an amide structure. It plays a crucial role in connecting two successive alpha-amino acids within a peptide or protein chain. Specifically, it forms between the carbon atom designated as C1 in one alpha-amino acid and the nitrogen atom labeled as N2 in the next one. This linkage is integral to the structure of a peptide or protein, ensuring the linear arrangement of amino acids along the chain.
Is There One Peptide Bond In A Dipeptide?
Is there only one peptide bond in a dipeptide? A dipeptide is formed by the linkage of two amino acids through a process called dehydration condensation, which involves the formation of a single peptide bond. The figure provided above illustrates the step-by-step process of dipeptide formation, helping to clarify the concept.
Discover 17 Where is the peptide bond located in this dipeptide

Categories: Collect 39 Where Is The Peptide Bond Located In This Dipeptide
See more here: tfvp.org
Peptide bonds form between the carboxyl group of amino acid 1 and amino group of amino acid 2.1: Amino acids undergo condensation to form a molecule called a dipeptide. The C−N bond is called a peptide bond. A peptide bond is the amide bond that occurs between the amine nitrogen of one amino acid and the carboxyl carbon of another amino acid. The resulting molecule is called a dipeptide.A peptide bond is basically an amide-type of the covalent chemical bond. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. This linkage is found along a peptide or protein chain.
Learn more about the topic Where is the peptide bond located in this dipeptide.
- Dipeptide Definition, Structure & Examples – Study.com
- 13.2: Peptides – Chemistry LibreTexts
- Peptide Bond – Definition, Formation, Structure, Examples
- A dipeptide has two peptide bonds. – Toppr
- The correct statements about peptides are: – BYJU’S
- How do you Identify a Peptide Bond? – BYJU’S
See more: blog https://tfvp.org/category/science