Why Does Octane Boil At A Higher Temperature Than Butane?
Explain Why Propanol Has Higher Boiling Point Than That Of The Hydrocarbon Butane?
Keywords searched by users: Why does octane have a higher boiling point than butane melting point of butane, octane boiling point, why does hexane have a higher boiling point than methanol, c4h10 boiling point, ch4 boiling point, when the process of condensation occurs, the kinetic energy of particles, a gaseous substance turns directly into a solid. which term describes this change?, which commercial technology commonly uses plasmas?
Why Is The Boiling Point Of Octane Higher Than Butane?
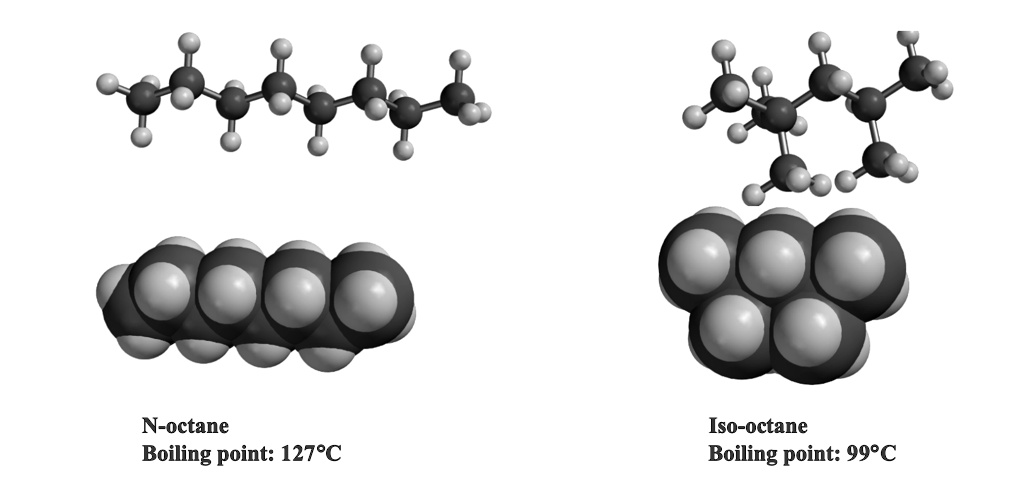
Why does octane have a higher boiling point than butane? The boiling points of hydrocarbons depend on their molecular structure and the forces holding their molecules together. In the case of 2,2,3,3-Tetramethylbutane, its molecular structure is more spherical compared to n-octane. This spherical shape reduces the efficient surface area available for intermolecular interactions compared to the larger, more linear structure of n-octane. As a result, 2,2,3,3-Tetramethylbutane has a lower boiling point than n-octane, primarily because its spherical shape limits the strength of intermolecular forces, such as Van der Waals forces, which play a crucial role in determining boiling points. So, in essence, the higher boiling point of n-octane can be attributed to its greater surface area and stronger intermolecular forces compared to 2,2,3,3-Tetramethylbutane.
Does Butane Or Octane Have A Higher Boiling Point?
Which substance, butane or octane, possesses a higher boiling point? Octane is known to have a higher boiling point than 2,2,3,3‑tetramethylbutane. This is attributed to the structural differences between the two compounds. Octane has a straight-chain structure with fewer branches compared to 2,2,3,3‑tetramethylbutane, which results in a larger “surface area” for octane. Consequently, octane exhibits stronger van der Waals forces between its molecules. This enhanced intermolecular attraction contributes to its higher boiling point. This information was last verified on January 28, 2023.
Why Does Butane Have A Lower Boiling Point Than Octane?
B. The reason butane has a lower boiling point than octane is primarily due to differences in their molecular structures. Butane consists of four carbon atoms and ten hydrogen atoms, whereas octane has eight carbon atoms and eighteen hydrogen atoms. This discrepancy in size and complexity influences the strength of intermolecular attractions between molecules. In the case of butane, there are relatively fewer sites for intermolecular attractions compared to octane. This lower molecular complexity results in weaker Van der Waals forces and a lower boiling point for butane. To elaborate further, the larger and more complex octane molecules have a greater number of electrons and a larger surface area, which allows for stronger Van der Waals forces to hold them together. In contrast, the simpler and smaller butane molecules experience weaker intermolecular forces, making it easier for them to transition from the liquid to the gaseous state, hence their lower boiling point.
Top 9 Why does octane have a higher boiling point than butane





Categories: Update 79 Why Does Octane Have A Higher Boiling Point Than Butane
See more here: tfvp.org

n-Butane. Hint: n-Octane is a straight-chain exacerbate that has a very large surface area. Along these lines, there are more van der Waals forces of attraction, bringing about high boiling points.2,2,3,3-Tetramethylbutane is more spherical than n-octane. Efficient surface area is more diffult than in the case of n-octane. Hence, its boiling point is lower than n-octane, which has a larger surface area.Octane will have a higher boiling point than 2,2,3,3‑tetramethylbutane, because it branches less than 2,2,3,3‑tetramethylbutane, and therefore has a larger “surface area” and more van der Waals forces.
Learn more about the topic Why does octane have a higher boiling point than butane.
- The highest boiling point is expected for: a.) Isooctane b.) n …
- n-Octane vs. 2,2,3,3-Tetramethylbutane
- 3.5: Properties of Alkanes – Chemistry LibreTexts
- 4.07.03 Prac Properties of Hydrocarbons Flashcards | Quizlet
- Which would you expect to have the higher melting point(or …
- Premium vs. Regular Gas – Progressive
See more: blog https://tfvp.org/category/science