Why Is Kc Affected By Temperature: Unveiling The Thermodynamic Mystery
19 Only Temperature Affects Kc
Keywords searched by users: Why is KC affected by temperature how does temperature affect kc, is kc affected by concentration, is kp affected by temperature, why does temperature affect kc but not pressure, why is kc not affected by pressure, is kc affected by pressure, why is kc not affected by concentration, is kc only affected by temperature
How Does Temperature Affect Kc?
The impact of temperature on the equilibrium constant, Kc, is crucial to understand in chemical reactions. Kc values vary with temperature changes, and these variations are linked to whether the reaction shifts towards the product side (right) or the reactant side (left). Specifically, when the temperature is increased in an endothermic reaction (where heat is absorbed), Kc tends to increase, favoring the forward reaction. Conversely, if the temperature is decreased in an endothermic reaction, Kc decreases, pushing the equilibrium position towards the reactants. In contrast, for exothermic reactions (where heat is released), an increase in temperature leads to a decrease in Kc, favoring the reverse reaction, while a decrease in temperature results in an increase in Kc, promoting the formation of products. Understanding these temperature-dependent changes in Kc is essential for predicting and controlling chemical reactions.
How Does Temperature Affect Equilibrium Constant K?
The equilibrium constant, denoted as K, is significantly influenced by temperature changes in chemical reactions. The impact of temperature on K varies depending on whether the reaction is endothermic or exothermic. In an endothermic reaction, raising the temperature leads to an increase in the value of K. This occurs because the higher temperature promotes the formation of more product molecules, shifting the equilibrium towards the right side of the reaction. Conversely, in an exothermic reaction, increasing the temperature causes a decrease in K. This shift happens because the elevated temperature favors the formation of reactants, thus pushing the equilibrium towards the left side of the reaction. Understanding this temperature-dependent relationship with K is crucial for predicting and controlling chemical equilibria in various processes. [Published on: 29th January 2021]
Why Does Temperature Affect Kc And Kp?
The impact of temperature on equilibrium constants, such as KC and KP, is a phenomenon closely linked to the principles of chemical reactions. When the temperature of a chemical reaction is altered, it has a significant effect on the equilibrium constant KP, as well as KC. This occurs because temperature alterations can lead to variations in the quantity of product generated within the reaction. Consequently, these shifts in product formation result in changes in the number of moles present on either side of the chemical equation. Consequently, this causes adjustments in the mole fractions of the reactants, which, in turn, influence the partial pressure of each reactant involved in the equilibrium. In summary, temperature fluctuations in a chemical reaction not only impact the extent of product formation but also lead to changes in the composition of the reacting system, thus affecting the equilibrium constants KC and KP.
Share 41 Why is KC affected by temperature



Categories: Aggregate 95 Why Is Kc Affected By Temperature
See more here: tfvp.org

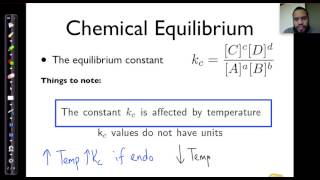
Temperature affects Kc because the forward reaction is either exothermic or endothermic.Changes in Temperature
Kc is larger when the reaction shifts right. This occurs if T is increased for an Endothermic Reaction or T is decreased for an Exothermic reaction. Kc is smaller when the reaction shifts left. This occurs if T is decreased for an Endothermic Reaction or T is increased for an Exothermic reaction.The temperature will affect the value of K. If the temperature is increased in an endothermic reaction, K will increase but in an exothermic reaction K will decrease. This is because by raising the temperature in an endothermic reaction, you are favoring the creation of the products for that reaction.
Learn more about the topic Why is KC affected by temperature.
- Why does only Temp affect K? – CHEMISTRY COMMUNITY
- Chapter 14. CHEMICAL EQUILIBRIUM
- How Does Temperature Affect the Equilibrium Constant?
- Changing Kp (A-Level Chemistry) – Study Mind
- equilibrium constants and Le Chatelier’s Principle – Chemguide
- Calculations with Equilibrium Constants (A-Level Chemistry)
See more: https://tfvp.org/category/science blog